Describe How a Sodium Ion Differs From a Sodium Atom
Many students have trouble in knowing the differences between a sodium atom and sodium ion. Sodium atom is neutral whereas sodium ion is a charged specie with a charge of 1The number of protons and electrons in sodium atom is same ie 11 whereas the number of protons in sodium ion 11 is more than number of electrons 10The size of sodium ion is smaller than sodium atom.
Because the extra electron forms a 3s2 valence electron configuration while the nuclear charge remains the same the Na ion is bigger than the parent.
. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. The sodium atom has only one electron in its valence shell. It has a role as a human metabolite and a cofactor.
The sodium ion has an extra positive charge shown by the sign. The main difference between these two particles of sodium is the composition of electrons in its orbits. It is formed when sodium atom gives up one electron.
Adobe stock Whereas an atom of Sodium and Chlorine form a compound called as hydrochloric acid HCl. The number of atoms of each element is the same on each side of the equation. In sodium atom there are 11 proton and 11 electrons ie.
In other words the valence shell last shell of sodium atom has only one electron. Because the 3s1 electron has been eliminated the Na ion is substantially smaller than the neutral Na atom resulting in a closed shell with n 2. Sodium ion has 10 electrons as sodium atom loses one electron to form sodium ion.
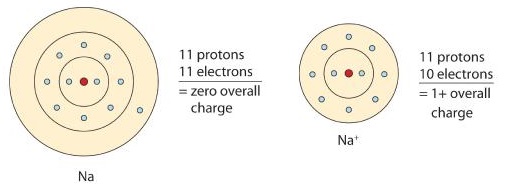
Sodium atom has 11 electrons. Sodium ion is positively charged. Now let us discuss sodium ion in sodium ion the atomic number of sodium is 11 and we know that there is no difference in the number of protons even when ion is formed.
Each one has 11 protons. In sodium ion there are 11 protons but 10 electrons. Atoms form ions due to loss or gain of electrons Image by.
The sodium atom is very reactive. Me as the number of products. There will be a one-to-one correspondence between protons and electrons.
The size of sodium atom is bigger than that a sodium ion. It has 11 protons but 10 electrons. Ions are charged particles.
Difference between sodium atom and sodium ion. Both ions now have the electron configuration of. Sodium is another atom which forms a metal.
Only sodium atom react vigorously with water whereas sodium ion does not. The state of matter is the same on each side of the equationWhich details from the passage are emphasized in both the text and images. A sodium ion differs from a sodium atom in that the sodium ion has a missing electron electron.
Is a sodium ion larger than a. It has 11 protons and 11 electrons. Ions are atoms which are devoid of few electrons or have few additional electrons.
These two are the basics particles of sodium which is a member of the First Group of the Periodic Table. There are distinct differences between an atom and an ion. It has a positive charge as opposed to the atom which is neutral.
Other Apps -. The charge on the ion can also be shown as and the electron structure. Sodium atoms are electrically neutral so the number of protons is equal to the number of electrons and thus we can say that there are 11 electrons as well in sodium atoms.
Sodium-ion is positively charged. Size of a sodium atom is larger than a sodium ion. A sodium atom contains 11 electrons with two electrons in the first.
Describe How a Sodium Ion Differs From a Sodium Atom Get link. The chlorine atom accepts the electron into the half-filled 3p orbital to give a chloride ion. In sodium ion there are 11 protons but 10 electrons ie sodium ion contains lesser number of electrons.
While an atom of Sodium and chlorine form sodium chloride. An atom can be an ion but not all ions are atoms. All group 1 metals will form a 1 ion when they react with non-metals.
The sodium atom loses its outer electron to become a sodium ion. Which of the following pairs of atoms would be likely to form an ionic bond. Nonpolar - the electrons are shared equally.
The sodium ion reacts faster than sodium atom. Its electronic configuration is 281it has 1 free electron in its valence shell which makes it highly reactive. How are a sodium atom and a sodium ion different.
What is the difference between a polar covalent bond and a non polar covalent bond. The sodium ion differs from the sodium atom in that it lacks its outermost electron. Atoms where the electrons and protons are not equal are called ions.
Sodium-ion has 8 electrons in its valence shell. The sodium ion has the same electronic structure as the neon atom but the charge on its nucleus is different from that on the neon atom. It reacts with air or water readily.
Sodium ion is a natural product found in Phytelephas aequatorialis Montanoa frutescens and other organisms with data available. An atom is a neutral particle an ion is a charged particleTaking a sodium 23 Na and sodium ion Na as an exampleFirstly we need to break down both into their subatomic particles protons neutrons and electronsThe sodium atom has-11 protons I know this from the atomic number of the periodic table-12 neutrons mass number is 23 23-11 12-11 electrons. An ion of sodium or any other element will have an unequal.
Polar - the electrons are shared unequally. They can be either positively charged ions or negatively charged ions. An equal number of protons and electrons.
But in sodium ion the last shell has 8 electrons. Now since we know sodium is. The compounds are the same on each side of the reaction.
Therefore sodium ion has one electron less than the sodium ion. It is an alkali metal cation an elemental sodium a monovalent inorganic cation and a monoatomic. A sodium atom has only one electron in its valence shell.
Atomic Structure Of The Sodium Atom Na Youtube 3 2 Ions Chemistry Libretexts 5 1 Ionic And Molecular Compounds Introductory Chemistry Ionic Bonds Biology For Majors I. The sodium ion still has 11 protons 11 positive charges but now only 10 electrons 10 negative charges. Molecules are groups of two or more atoms that are chemically bonded.
If an atom contains maximum number of electrons than protons then it is called anion. If an atom is charged electrically it is considered an ion. Sodium 1 is a monoatomic monocation obtained from sodium.
Thus a sodium atom 1 s 22s 22p 63s 1 may lose the electron occupying the 3s orbital giving a sodium ion. Sodium ion has a 1 charge whereas sodium atom is. It tends to react with other atoms and ions therefore it behaves differently than does a sodium atom.
A neutral atom of sodium will have 11 electrons around it. The atomic number of sodium is 11. Sodium ion has obtained a stable electronic configuration by giving out one electron from the sodium atom.
1 12 Ions Losing And Gaining Electrons Chemistry Libretexts
Why Does The Na Ion Have A Smaller Radius Than The Na Atom Quora


0 Response to "Describe How a Sodium Ion Differs From a Sodium Atom"
Post a Comment